In November 2022, our valued subsidiary DiaSys Diagnostics India participated in the 48th Annual Conference of Association of Clinical Biochemists of India (ACBICON) in Delhi. The product portfolio of DiaSys India was presented at the booth on three intensive days.
DiaSys at ACBICON 2022 in Delhi, India

During the conference, Ms. Isabella Wieland, Global Product Manager for PCT from the DiaSys Headquarter had the opportunity to hold an industry-sponsored workshop titled “New PETIA for Procalcitonin - Routine experience and lessons learned from EQA evaluations” which featured Procalcitonin FS, DiaSys’ new particle-enhanced immunoturbidimetric assay (PETIA) to determine procalcitonin (PCT).
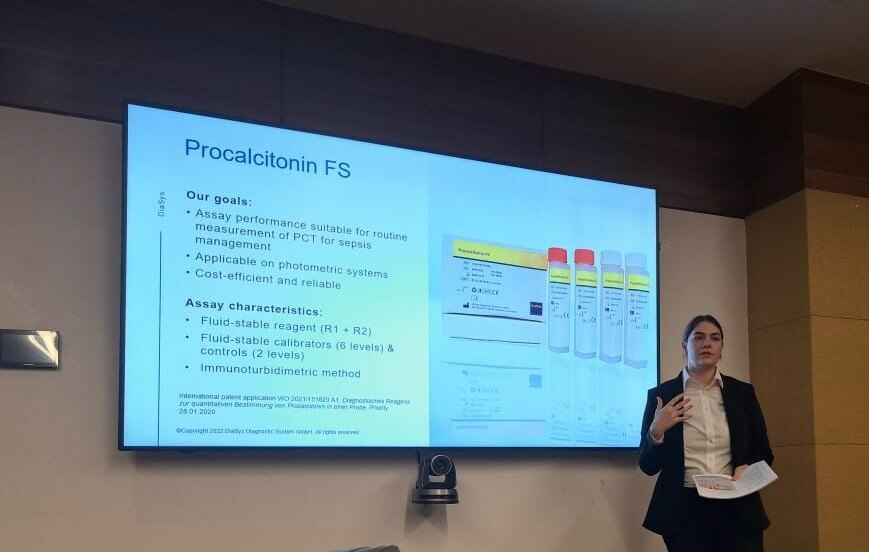
After an introduction to sepsis diagnosis and management as well as general information on the parameter PCT, results from German External Quality Assessment (EQAs) schemes were shown. Furthermore, DiaSys’ double approach to the challenging market situation was presented:
1. As an active member of the IFCC working group on standardization of PCT, DiaSys contributes to establishing a higher order reference method for quantification of PCT by stable isotope mass spectrometry.
2. DiaSys offers a cost-efficient and reliable assay that can be applied to various common clinical chemistry analyzers, making the assay broadly available to laboratories worldwide.
The workshop provided a platform for colleagues and interested professionals from different disciplines to exchange and share their knowledge, experience and ideas on how to implement the new PETIA test in clinical practice.
We thank all visitors for the consistently positive feedback and the many interesting discussions.

 Isabella Wieland
Isabella Wieland